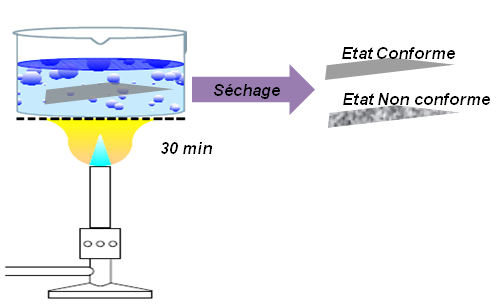
Gravimetry
UV and low-angled light
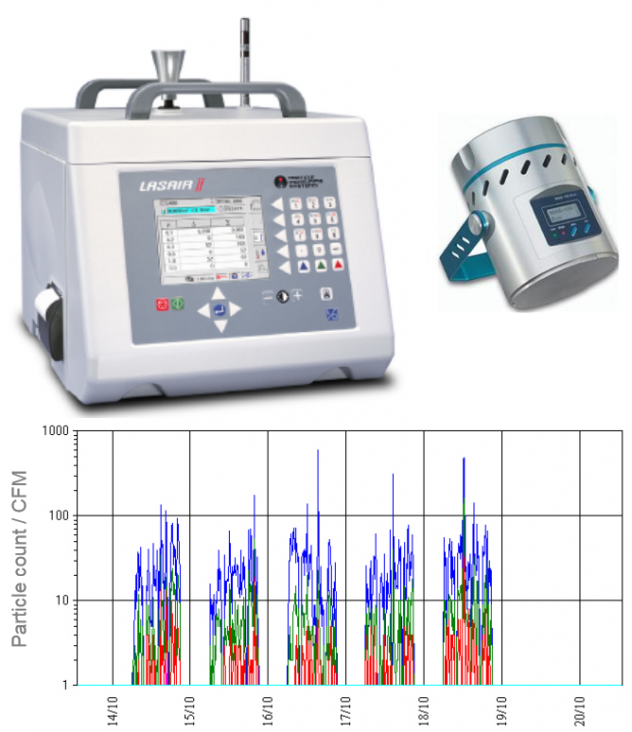
Liquid Particle Counting (LPC)
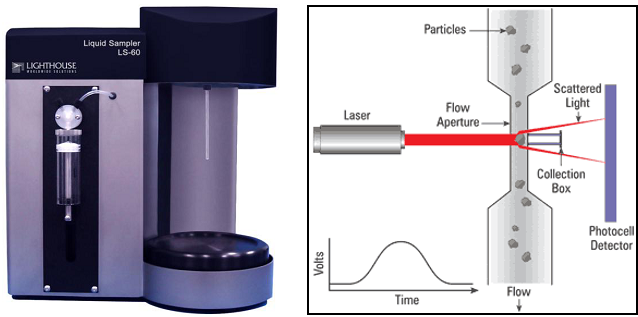
The particle contamination of the part to be controlled is extracted with DI water (18 Mohms, filtered at 0,2 µm + ultrasonics, shaking table, …).
A sample of the extracted solution is analyzed with a LPC counting system. The difference between the number of particles from the blank solution and the extracted solution indicates the level of cleanliness of the product.
Granulometry
Gravimetric membranes are scanned and then analyzed by a specific imaging software. This tool is a fast and reliable solution to have the complete cartography of particles and fibers (their number and size) and their properties (size limit > 5 µm).
Ionic contamination
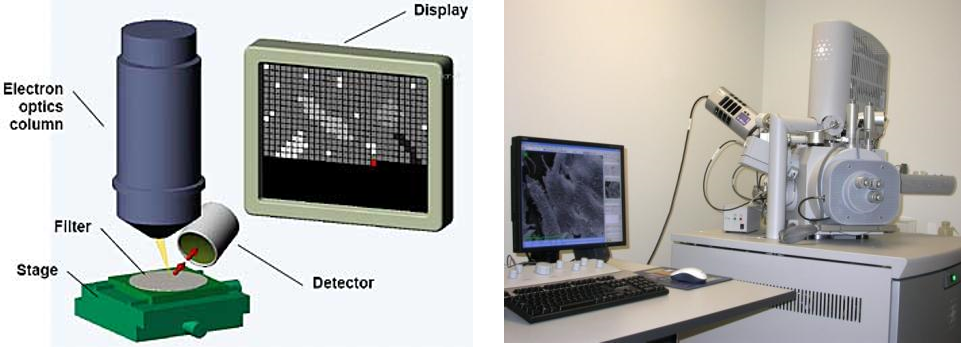
Analysis by SEM-EDX
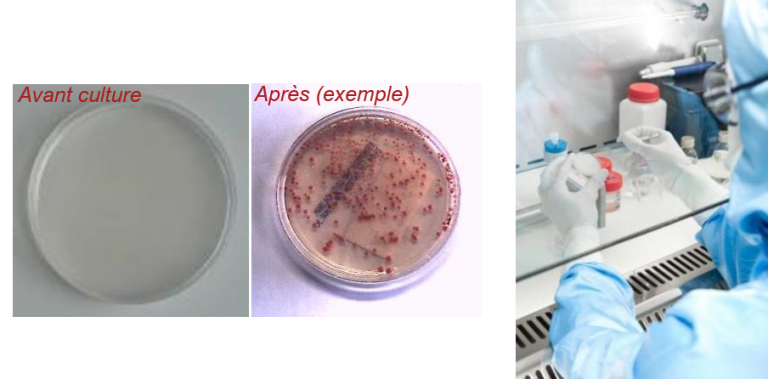
Microbiological controls
Packaging sealing validation
Tracking of a specific contaminant
Prove that a specific contaminant is no more present on the product after the cleaning process. This method is similar to the tracking of surfactant trace after detection of the type of tracer of he contaminant: ionic, metallic, organic,…
Non Volatil organic residue control
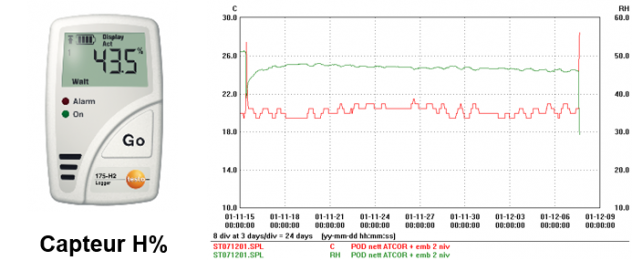
Humidity control
Metallic contamination
Organic contamination
Sterility test of solutions